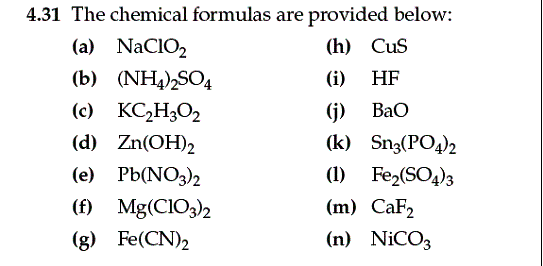
Chapter
4 Answers
4.1 (a)
Compound (b) Element (c)
Mixture
(d) Mixture (e) Compound (f) Element
(g) Element (h) Compound (i) Compound
4.2 The names of the elements are provided below:
H hydrogen He helium Ne neon
Al aluminum S sulfur Cl chlorine
O oxygen F fluorine Mg magnesium
Mn manganese Pb lead Br bromine
Cr chromium Ag silver Si silicon
Na sodium K potassium Cu copper
Fe iron Ca calcium Ba barium
Zn zinc Co cobalt
4.3 (a) C (b) F (c) Na
(d) Pb (e) Al (f) Ba
(g) Cl (h) Li (i) Zn
(j) Mg (k) Ni (l) Fe
4.4 Table 4.1 reveals that oxygen, silicon, aluminum, and iron are the four
most abundant elements in Earth’s crust, oceans, and the atmosphere.
4.5 (a) Chemical change (b) Chemical change (c) Chemical change
(d) Physical change (e) Physical change (f) Physical change
4.8 Strontium has four naturally occurring isotopes. In an average sample
of strontium atoms, 0.50% are strontium-84 atoms, 9.90% are strontium-86 atoms,
7.00% are strontium-87 atoms, and 82.60% are strontium-88 atoms. The atomic
weight of strontium is calculated as follows:
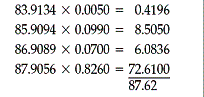
4.9 Nitrogen, phosphorus, and arsenic possess similar chemical properties,
because they are members of the same family, Group VA.
4.10 (a) Rn (b) Se (c) Zr (d) Er, Tb, Y, and Yb
4.12 (a) Am is the symbol for americium.
(b) Pu and Cm are the symbols of plutonium and curium, the two elements
that occupy positions on each side of americium on the periodic table.
(c) Collectively, the elements having atomic numbers from 89 to 103 occupy
a position to the left of the bold line in Figure 4.3. Consequently, americium
is a metal.
(d) Collectively, the elements having atomic numbers from 89 to 103 are in
the same family as scandium. Consequently, americium is likely to possess
chemical properties similar to those of scandium.
4.14 (a) Cesium and strontium are members of the alkali metal and alkaline earth
metal families,
respectively.
(b) Since sodium, potassium, and cesium are members of the same family,
each is likely to concentrate in the body’s tissues. Since calcium and
strontium are members of the same family,
they are likely to concentrate in the body’s
bones.
4.15 (a)
1 (b) 4 (c) 4
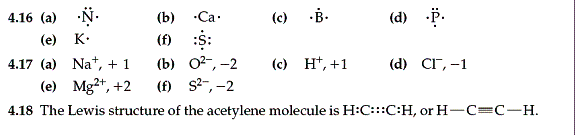
![]()
4.26 The chemical formulas of magnesium hydroxide and aluminum hydroxide
are Mg(OH)2 and Al (OH)3 respectively.
4.28 The chemical formula of ammonium nitrate is . NH4NO3
4.30 The name of the substance having the chemical formula P4S3 is tetraphosphorus trisulfide.